In the last article in the series on crude oil, we learned where oil comes from. In this edition, we’ll discuss how fuels, lubricants, and other petroleum products are made from oil.
After oil is extracted from the earth, it is separated into different components roughly according to how big the molecules are. Differences in boiling points (which are highly dependent on hydrocarbon chain length) allow for the separation of different components from one another.
Distillation is the most common process. If you want to take an alcohol mixture that’s 20% alcohol and 80% water and make it more concentrated you can heat it up so that the alcohol vaporizes while most of the water stays as a liquid. If you capture this alcohol vapor and condense it back into a liquid, it will have a higher concentration of alcohol. There’s no chemical reaction.
A refinery basically separates crude oil into different fractions. The heaviest fractions, like asphalt, have very long carbon chains and are actually solids while shorter chains are light and are usually gases like natural gas. [If you skipped the article on hydrocarbons, go back and check it out.] In between these two extremes are liquid hydrocarbons which are the main components of gasoline, kerosene, heating oil etc. The picture below shows how crude enters a fractionating column and is separated into light and heavy components by chain lengths.
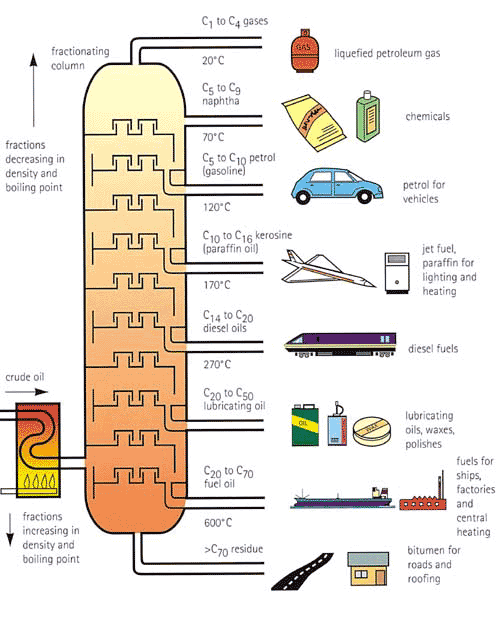
The crude oil comes in to the tower on the left after it is heated in a furnace. The fractional distillation process occurs inside the tower and the lighter components rise to the top. The outlets on the right side are labeled by what sort of products they become, by the temperature at which they transition from a liquid to a vapor (aka boiling point), and by the range of carbon atoms in the stream. Remember that crude oil is made of hydrocarbons which are chains of carbon atoms bonded together with hydrogen atoms around the outside. At the top carbon chains from 1 to 4 carbon atoms long come out of the tower. At the bottom, much longer chains (longer than 70 atoms long) are removed from the tower.
After the components have been roughly separated, the sulfur and other contaminants are removed (we’ll discuss this a bit more in another article). Then some components are ready to be sold as finished products. Others need to be separated even more before they can be sold so the same distillation process is repeated. Different fractions have different uses. Below is a short list of products that come from crude oil with little alteration:
- Petroleum coke
- Sulfur
- Asphalt
- Waxes
- Lubricants
- Alkenes (olefins, which are polymerized into plastics)
- Fuel oils
- Jet fuel
- Diesel
- Gasoline
- Kerosene
- Aromatics (used in chemical production)
- Lights ends (ethane, propane, butane, pentane and other short chain hydrocarbons)
- Natural gas
Some refineries, called high conversion refineries, take the really heavy, long chain molecules and break them into smaller pieces using a technique called cracking. Basically, the molecules are heated until they become unstable and break apart. These smaller pieces are then sorted and combined with other similar molecules. Refineries do this because the lighter products are generally worth more than the heavier products. For example, would you rather have 100 pounds of gasoline (C5-C10) or 100 pounds of asphalt (C70+)? What if I told you that gas was worth 10 times what asphalt is worth?

These high conversion refineries have more advanced and expensive equipment but can buy heavy, sour (and therefore cheaper) crude and sell more expensive products like gasoline, lubes, and jet fuel instead of asphalt. A barrel of crude oil is split into the following components (as a percentage of the crude barrel):
47% – Gasoline for use in automobiles
23% – Heating oil and diesel fuel
18% – Other products, which includes petrochemical feedstock—products derived from petroleum principally for the manufacturing of chemicals, synthetic rubber and plastics
10% – Jet fuel
4% – Propane
3% – Asphalt
The percents add up to more than 100% because smaller cracked molecules take up more space than the original, long, and uncracked molecules. [Numbers from the Department of Energy FAQ on crude oil]
These products all fall into two broad categories: energy products and non-energy products (like: ink, crayons, bubble gum, dishwashing liquids, deodorant, eyeglasses, records, tires, ammonia, and heart valves [DOE FAQ]). It’s easy to see how important crude oil is since it’s the precursor to so many important things in our daily lives.
The Bottom Line: Crude is separated into different fractions by a process called distillation based on the length of the hydrocarbon molecules. The harmful contaminants are removed from these fractions and they are either sold as finished products or undergo further processing. By refining heavier, sour crudes, US refineries make more money than by processing light sweet crudes.

