This article is about carbon and how it moves around our planet. Carbon is the fourth most abundant element in the universe and the basic building block for all life. Carbon atoms are everywhere – the stone we walk on, the CO2 our bodies produce and release into the air, the backbone of polymers, and most importantly, carbon is the foundation of all life.
The total amount of carbon on the earth is essentially constant. Imagine a log of wood inside of a large sealed metal box filled with air. Weigh the entire box and its contents. Now cut the log up until it is just a pile of sawdust. Weigh the box again – it has the same weight. Now burn the sawdust inside until all that is left is a pile of ash inside a smoke filled box. Weigh the box again. The weight is the same as before.
Mass is not created or destroyed when you cut the log up or burn it. It simply changes form. If you were to uniquely label each atom in the original log, you would still be able to find every atom after the log was cut up and burned. The atoms would be arranged differently but they would all be somewhere in the box. The image below shows how a molecule of methane reacts with oxygen to form carbon dioxide and water. Energy is also released in this process which can do work for us. Click here to learn more about hydrocarbons like crude oil and coal.
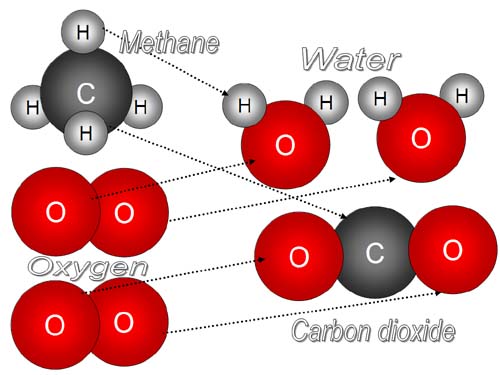
The box example applies to the earth too. Mass doesn’t enter or leave earth unless we’re hit by something or we send a satellite into space. So if the number of carbon atoms on the planet is constant no matter what we do, then why is there suddenly a “carbon problem?” The problem is that we are converting a lot of carbon from one form to another.
When we burn fossil fuels we don’t create carbon, we just convert it from a hydrocarbon – which was previously buried under the earth’s surface – to CO2 in the atmosphere. CO2 is a greenhouse gas which means that it prevents heat from leaving the earth. If there was too much CO2 in the atmosphere, then the planet would trap too much of the sun’s energy on the surface and life would die off because of the heat.
Fortunately, the earth also has systems to take up CO2 to avoid it building up in the atmosphere. Venus’ atmosphere is over 95% CO2 and this contributes to the lead-melting temperatures on the surface of Venus. [Venus is also closer to the sun than the earth which means it receives more of the sun’s energy. But the CO2 is a very important factor: Mercury which is closer to the sun than both Venus and Earth, never has temperatures as high as Venus’ because there’s hardly any CO2 in Mercury’s thin atmosphere. This thin atmosphere doesn’t trap any heat on the surface of the planet which means that nights on Mercury are extremely cold. Enough about other planets – back to carbon on earth.
There are several systems that take up CO2 on the earth. Plants, for example, take CO2 from the air and make sugars by a process called photosynthesis. Light energy from the sun is converted into chemical energy, which the plant can use later to grow or reproduce. If this plant is eaten by humans, animals, or bacteria, then the carbon atoms that the plant took out of the air will be released as CO2 again when the organism uses the stored sugars to release energy. Plants take CO2 out of the atmosphere temporarily while they’re alive and then release it back when as they’re digested.
[By the way – when animals eat plants and then humans eat animals, very little of the original energy in the plant makes it to the human. This means it takes a lot of energy to feed animals to the point where they can be slaughtered and eaten. For more, check out Beef: Getting More than You Bargained For]
If the plant (or more commonly, little phytoplankton that live on the surface of the ocean) die, sink to the bottom of the ocean, and are preserved in the right way, then they can be transformed into a fossil fuel like crude oil over millions of years. This process of photosynthetic organisms taking CO2 out of the atmosphere and stores it for a long time. Over the past few 100 million years, a lot of CO2 was pulled out of the atmosphere and stored in the earth in the form of fossil fuel. Since they take so much time and energy to make, pockets of oil and coal are incredibly energy dense and useful to humans as a source of essentially free energy (if you think the price at the pump is expensive, imagine how much of your own time and energy it would cost you to push your car around wherever you went).
During the last century or two, humans have been finding and consuming a lot of fossil fuels to do useful work. Using all this energy releases the stored carbon that was taken out of the atmosphere a long time ago. The issue here is the shear scale of our carbon emissions. The graph below shows how CO2 levels have increased over the last 200 years since humans started burning a lot of fossil fuels. There’s a very clear upward trend over the last 150 years in CO2 levels in the atmosphere.
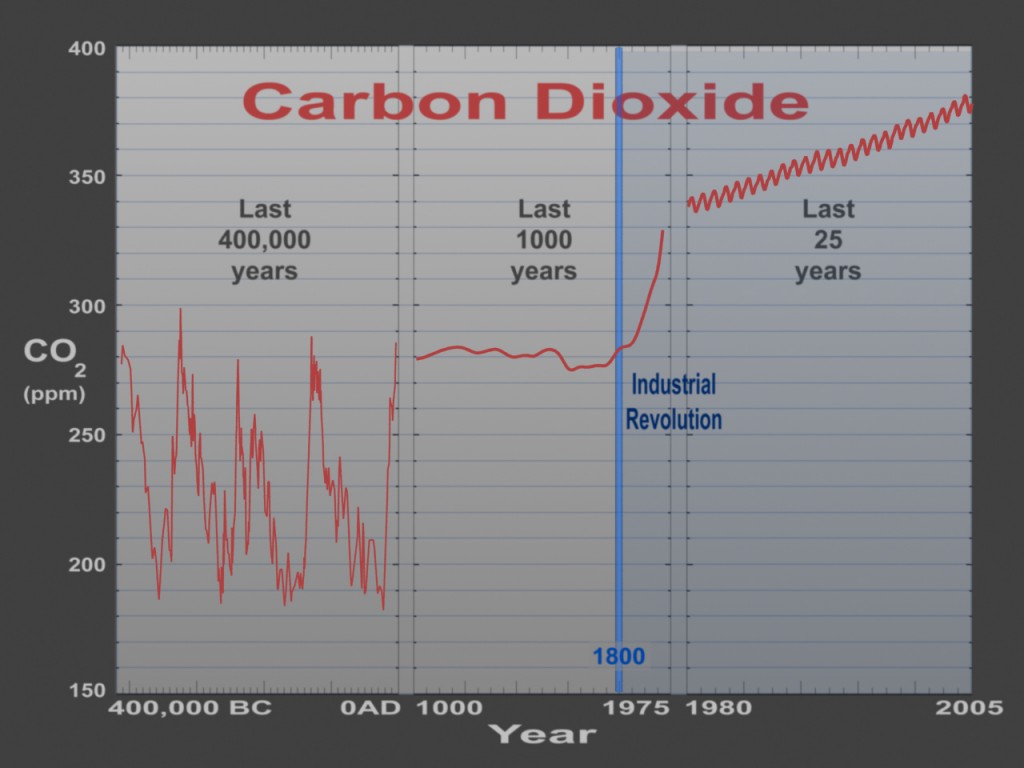
We are releasing carbon into the atmosphere faster than plants (and other carbon sinks) can pull it back out. This means that more and more CO2 is staying in the atmosphere – a fact that is warming the planet and contributing to climate change. The picture below is a simple diagram of the carbon cycle. The black labels and numbers shows the amount of carbon stored in various parts of the planet.
The deep oceans, for example, are the largest carbon sink, storing 38,100 gigatons of carbon. The purple arrows and numbers show how the carbon is moving around our planet. Humans release about 5.5 gigatons of carbon every year from fossil fuels alone. That’s about 40,300,000,000,000 pounds of CO2 a year, nearly enough gas to fill 1.8 Billion Goodyear blimps or cover the surface of the earth in CO2 filled ping-pong balls.
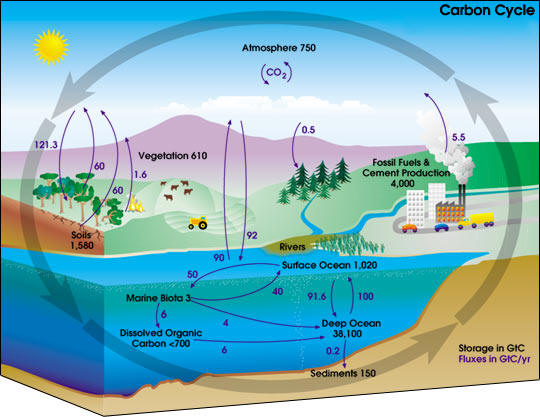
As CO2 concentrations in the atmosphere get higher, the average temperature of the earth rises. As the earth’s temperature rises, the oceans become warmer. Cold water can store more dissolved CO2 than warmer water so the oceans release even more CO2 into the atmosphere as they warm up. [Have you ever opened a warm coke before and it made a huge mess all over yourself? That’s because the CO2 that makes the coke fizzy was less soluble in the coke than if the coke had been cold]
Bottom Line: Who cares? You do – you just may not fully realize it yet. Currently, we’re releasing far more CO2 into the atmosphere than is being removed. 1.8 Billion Goodyear blimps filled with anything is a lot. The CO2 in the atmosphere will soon reach high enough levels to do serious permanent damage to the earth and its inhabitant (that includes humans). You wouldn’t give your child or grandchild a warm coke and let them deal with the sticky mess. So why would you give them a messy planet to clean up?

Good article on basic science that 5th graders understand, but the scientifically illiterate US populace does not understand or care to understand. Sometimes its good to refresh on basic ideas, like that there is a carbon cycle driven by the sun or that we are all currently living on a planet spinning in space.
Any one of these illustrations and graphs could debunk 99 percent of the “debates” and general discussions and commentary that are commonplace in the media and general conversation.
I’d like to see Lou Dobbs or Sean Hannity or Neil Cavuto or Glen Beck or any one of the pundits put up one of these graphs and go, “well, it turns out that coal fired power plants are not a natural cycle at all… Who knew?….
Instead, we see Exxon Mobil front groups misinforming the already misguided and illiterate and disinterested public.
If only science were as interesting as politics or power or wealth or fame…
Good website, keep it up.