As part of the series on Crude Oil, this article answers the question “What are Hydrocarbons?”
Hydrocarbons are combustible chains of carbon and hydrogen atoms. The backbone of these molecules are carbon atoms bonded together to form chains. Hydrogen atoms also bond to the carbon atoms but are never attached to anything else so they don’t form chains. These carbon chains can be anywhere from just a few carbon atoms long to thousands of carbons long.
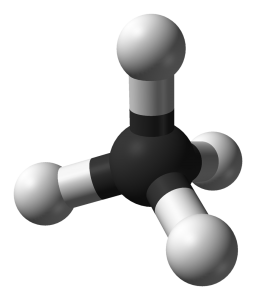
Hydrocarbon molecules with different lengths and configurations have different physical and chemical properties. For example, the smallest hydrocarbon is methane (pictured below) with just one carbon atom and four hydrogen atoms bonded around the lone carbon. Methane is the main component of what is commonly called natural gas. In the images below, the black atoms are carbon and the white atoms are hydrogen.
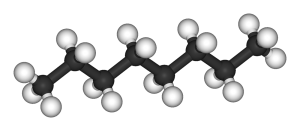
Octane, on the other hand, has eight carbon atoms bonded together and 18 hydrogen atoms bonded to these carbons. Octane can have several different arrangements beside the straight chain configuration (pictured below). These other arrangements of octane, called isomers, each with 8 carbon atoms and 18 hydrogens, have different properties because of their different structures.
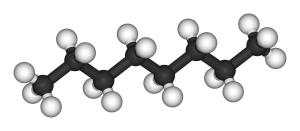
One of these isomers, iso-octane, is the basis for the octane scale that you may be familiar with when you fill up your car with gasoline. Molecules roughly similar to octane in length and structure make up major components of liquid fuels like gasoline and diesel.
Now picture a hydrocarbon molecule that is hundreds of carbon atoms long with dozens of long branches all along the main backbone and more complicated organic chemistry like aromatic rings and double bonds. The image below is what the average coal molecule might look like.
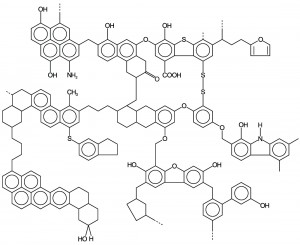
Even though each of these examples of hydrocarbons is made of the same basic building blocks, they have different physical properties. For example: Coal is a solid, gasoline is a liquid, and natural gas is a gas at the normal temperature and pressure conditions on the surface of the earth.
Even though these molecules are very different, when they are broken apart they recombine to form water and CO2 – the products of all combustion reactions. When water and CO2 form, they release energy. This energy can do useful work like heat water for a shower, cook food, or cool a home. Energy is really useful because of the work it can do. That’s why it’s important that we make sure we use energy carefully – so that we can continue to enjoy it’s benefits.